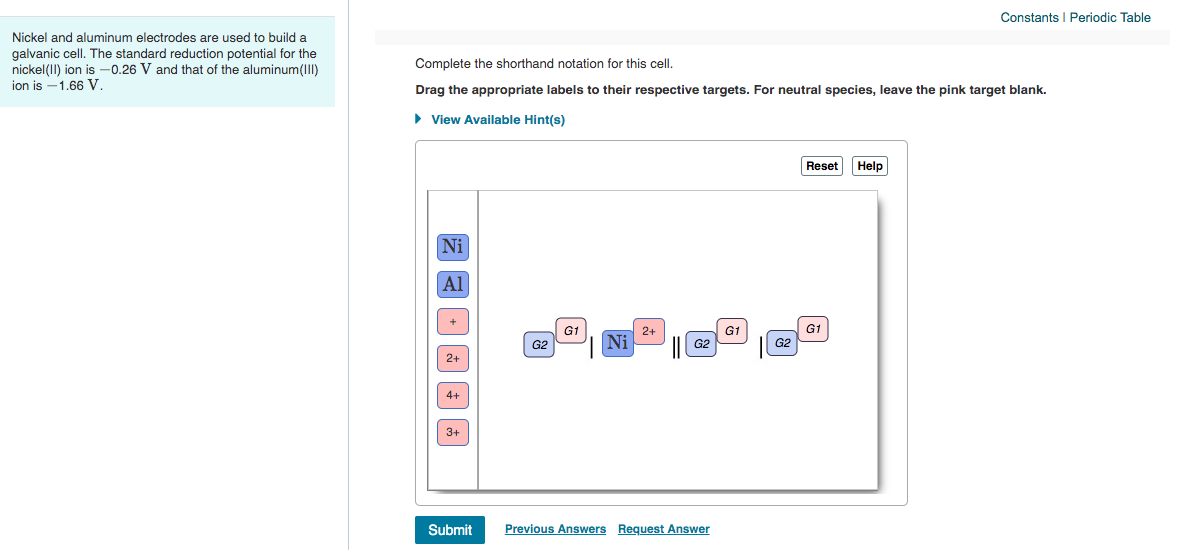
Complete the Shorthand Notation for This Cell
The electrode on the left is the anode and the one on the right is the cathode. The electrode on the left is the anode and the one on the right is the cathode They give you 2AgCl s.
Solved Constants Periodic Table Nickel And Aluminum Chegg Com
I put this but kept getting it wrong anybody knows how to fix it from here.
. C Calculate ÎGÂ for the cell under standard conditions. Nika Kononov 3E Posts. B Label the anode and cathode and indicate the direction of ion flow.
To calculate E cell you subtract the least positive electrode potential value from the most positive. Ni s - Ni 2 aq 2e. The electrode on the left is the anode and the one on the right is the cathode.
Chemistry Science Organic chemistry CHEM 113. Complete the half-reactions for the cell shown and show the correct shorthand notation for the cell. Also remember that in the shorthand cell notation no stoichiometry is shown at all - just species.
Complete the shorthand notation for this cell. The electrode on the left is the a. Achieve week 78 Q7.
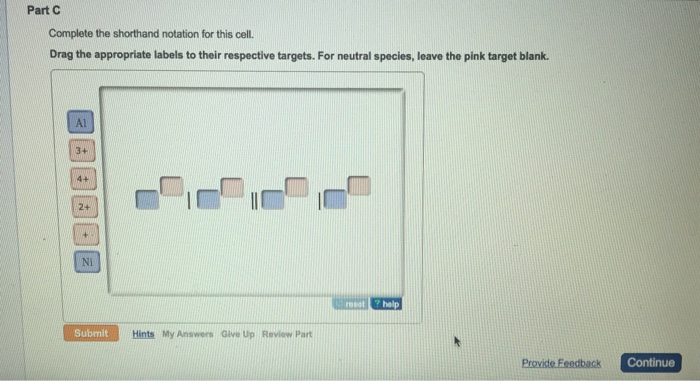
A Complete the drawing by adding any components essential for a functioning cell. Complete the half reactions for the cell shown here and show the shorthand notation for the cell by dragging labels to the correct position. Complete the halfreactions for the cell shown and show the shorthand notation for the cell.
Drag the appropriate labels to their respective targets. Complete the half-reactions for the cell shown and show the shorthand notation for the cell. For the cell shown in Figure 1 in Galvanic Cells the shorthand notation is cePts ceCl_2g mid ceCl1 M parallel ceFe21 M Fe31 M mid cePts nonumber According to the conventions we have just developed this corresponds to the cell reaction.
The cell anode and cathode half-cells are separated by two bars or. Cus 2 OH-2 CuOHs 2e- Co Cu Cathode half-reaction. The double vertical lines represent the salt bridge.
2 CI 2e Cathode half-reaction. Up to 256 cash back 18 Nov 2019. The electrode on the left is the anode and the one on the right is the cathode.
Does anyone know how to solve this problem. See full answer below. 2 AgCls 2e- Ags 2 Cl- Shorthand notation.
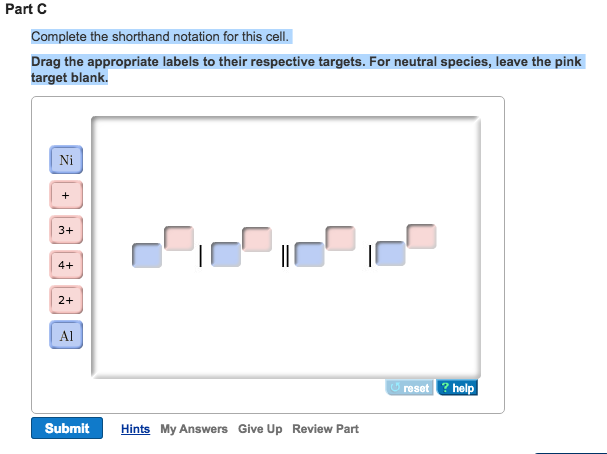
The electrode on the left is the anode and the one on the right is the cathode. For neutral species leave the pink target blank. In chemistry it is a shorthand way of expressing a certain reaction in an electrochemical cell.
The electrode on the left is the anode and the one on the right is the cathode. The left side of the cell shown is always to be written as an oxidation. The electrode on the left is the anode and the one on the right is the cathode.
2e 2CI Shorthand. Steps to write cell notation are as follows. CuOH2- -COOH2S KOHaq CuOHs Cus.
Complete the two reduction half reactions for the cell shown at the right and show the line notation for the cell by dragging labels to the correct position. Consider the following galvanic cell. The right side of the cell shown is always to be written as a reduction.
B Determine which electrode will function as the anode and which will function as the cathode when the cell runs under standard conditions. C Write a balanced equation for the cell reaction. Complete the halfreactions for the cell shown and show the shorthand notation for the cell.
AlsIgAl3 aqll4Ni2 aq4l5Nis Note the most oxidised form goes next to the salt bridge which is. The complete shorthand notation for this cell is. The electrode on the left is the anode and the one on the right is the cathode.
Complete the half-reactions for the cell shown and show the shorthand notation for the cell. Complete the half reactions for the cell shown here and show the shorthand notation for the cell by dragging labels to the correct position. Cos 2 COOH2s 2OH Shorthand notation.
Pbs 2 Cl- PbCls 2e- Cathode half-reaction. EqAlleft s right left Al3left aq right left right Ni2left aq. Pbs P6CI s CI aq CI aq AgCIs Ags Answer Bank 2 Ags 2 AgCls AgCls CI aq Pbs Ags PbCl s Incorrect.
Anode and cathode electrode in electrochemical cell is called as half cells. - Voiceover Before we get into shorthand notation lets review the structure of the Galvanic or Voltaic Cell. 2H aq 2e - H 2 g.
Consider the cellPts H2g Haq Cl aq Cl2g CsWhat is serving as the cathode in this electrochemical cellA. The two half cells are separated by double vertical lines. Complete the halfreactions for the cell shown and show the shorthand notation for the cell.
Complete the half reactions for the cell shown here and show the shorthand notation for the cell by dragging labels to the correct position. In the left side there is a solution of Pbcl2 s and it contains the elctrode pb and on the right side there is solution of AgCl S which contains the. They are a shorthand description of voltaic or galvanic spontaneous cells.
The electrode on the left is the anode and the one on the right is the cathode. E cell 025 1662 041 V. Determine the cell notation for the redox reaction given belowSns 2 Haq Sn2aq H2ga H2g Haq Pt ll Sn2aq Snsb Haq.
The shorthand notation is. The electrode on the left is the anode and the one on the right is the cathode. And so we already know what happens on this electrode on the left the Zinc electrode or the solid Zinc turns into Zinc 2 ions.
Remember a Voltaic Cell uses a spontaneous redox reaction to create an electric current. Cell notation or line notation is a shorthand description of voltaic or galvanic spontaneous cells. Cell notation is a shorthand hand notation to write redox reaction of a cell.
A Use shorthand notation to describe an electrochemical cell that contains silver Ag and copper Cu electrodes.

Solved Complete The Shorthand Notation For This Cell Drag Chegg Com

Kac32 8 Electrochemistry Cell Shorthand Notation Youtube
Solved Complete The Shorthand Notation For This Cell Drag Chegg Com
No comments for "Complete the Shorthand Notation for This Cell"
Post a Comment